Naming Alkanes Alkenes And Alkynes Ppt
Mar 02 2014 RULES FOR NAMING ALKANES If a chain has 2 or more different groups joined to it the groups are written in alphabetical order ie. Alkynes are named in the same general way that alkenes are named.
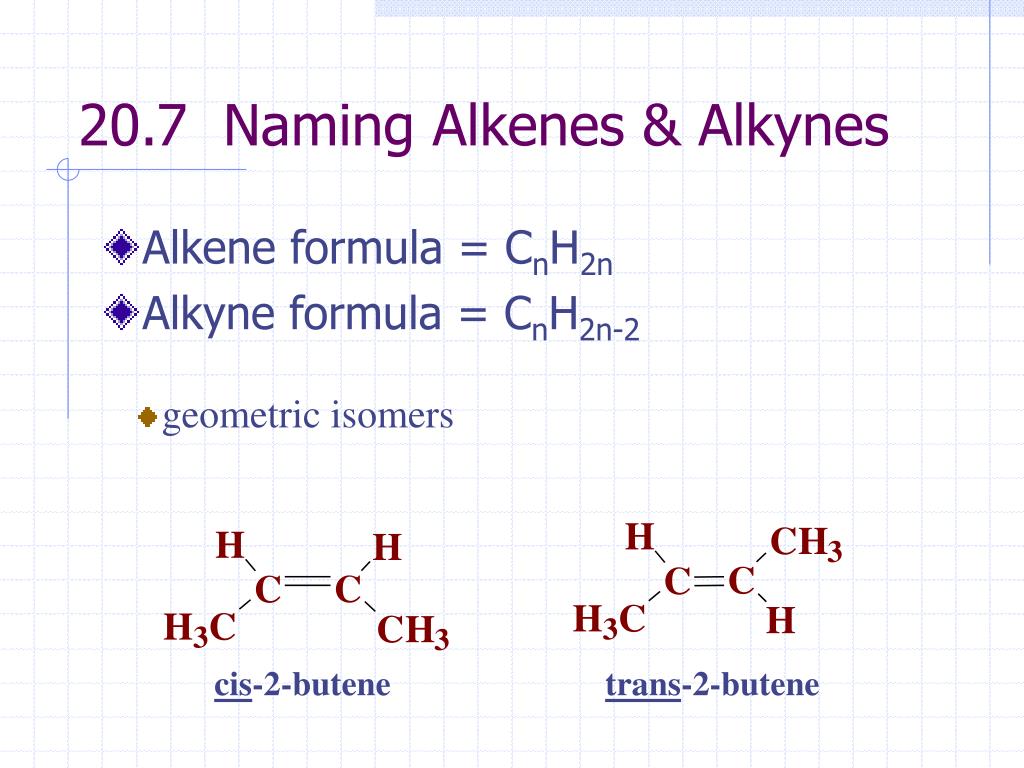
Ppt 20 7 Naming Alkenes Alkynes Powerpoint Presentation Free Download Id 3741371
Here is what we do.

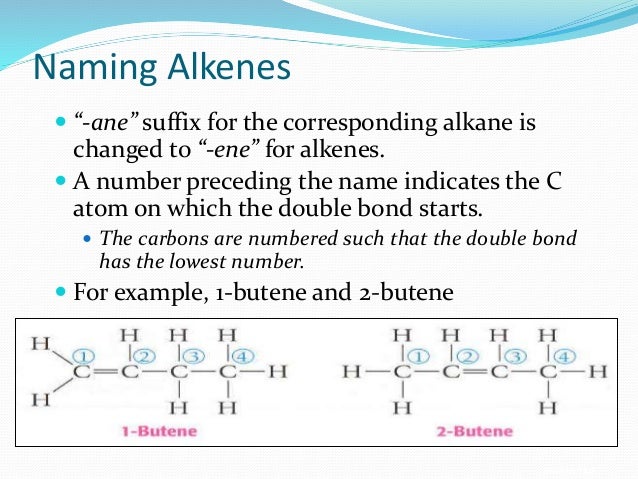
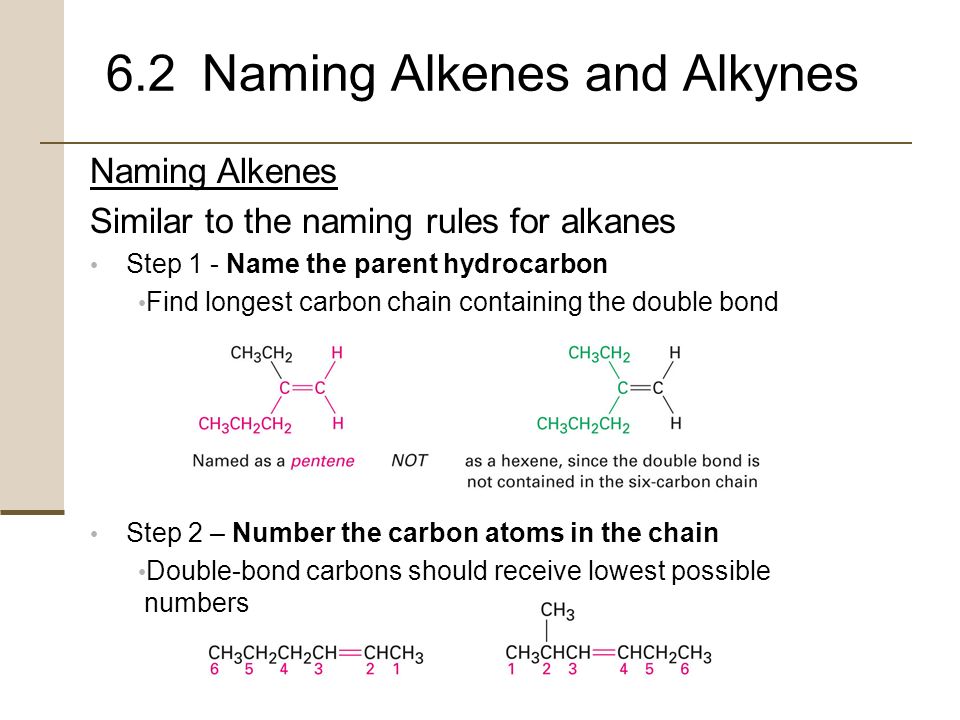
Naming alkanes alkenes and alkynes ppt. Apr 25 2012 For each alkane write the correct IUPAC name 1. Naming Alkenes and Alkynes Naming Alkenes Similar to the naming rules for alkanes Step 1 - Name the parent hydrocarbon Find longest carbon chain containing the double bond Step 2 Number the carbon atoms in the chain Double-bond carbons should receive lowest possible numbers Begin at end nearer first branch point if double bond is equidistant from the two ends. Many of the same rules for alkenes apply to alkynes.
The alkyne position is indicated by the number of the alkyne. Name the longest carbon chain with a double or triple bond Indicate the location of the double or triple bond in the main chain by number starting at the end closer to the double or triple bond Cycloalkenes do not require the numerical prefix but the bonds are given numbers 1 and 2 Give the location and. The IUPAC name for acetylene is ethyne.
-ene Many of the same rules for alkanes apply to alkenes 1Name the parent hydrocarbon by locating the longest carbon. Condensed Formula Name CH 4 methane CH 3 CH 3 ethane CH 3 CH 2 CH 3 propane CH 3 CH 2 CH 2 CH 3 butane CH 3 CH 2 CH 2 CH 2 CH 3 pentane. Naming and Drawing Alkynes and Cyclic Hydrocarbons.
Naming and Drawing Alkenes For each alkene write the correct IUPAC name. LecturePLUS Timberlake 4 Naming Alkenes and Alkynes When the carbon chain has 4 or more C atoms number the chain to give the lowest number to the double or triple bond. Alkanes Alkanes are often called saturated hydrocarbons Organic compounds composed of carbon and hydrogen that contain the largest possible number of hydrogen atoms per carbon atom.
You can view it all now for just More info. Okay so lets summarize the rules for naming alkenes and alkynes. This has been designated as a pay-to-view presentation by the person who uploaded it.
And this concludes its free preview. In the IUPAC system change the ane ending of the parent alkane name to the suffix yne. Ive already paid for.
Number the carbon chain from the end of the carbon nearest. Alkene Nomenclature please read and understand Prefix-Parent-Suffix Suffix for alkenes. ALKANES ALKENES ALKYNES AND CYCLOALKANES ARE HYDROCARBONS COMPOUNDS CONTAINING ONLY CARBON AND HYDROGEN.
PowerPoint PPT presentation free to view. Can include different types of hydrocarbons like pencil lead graphite and diamonds how they are both. Naming alkenes follows the same rules as before To show where the double bond is however you number the chain from the end closest to the double bond and then put the corresponding number before the ene in the name eg.
EACH OF THESE FORM A HOMOLOGOUS SERIES A GROUP OF ORGANIC COMPOUNDS HAVING A COMMON GENERAL FORMULA OR IN WHICH EACH. You must be able to name. The International Union of Pure and Applied Chemistry IUPAC names for alkynes parallel those of alkenes except that the family ending is - yne rather than - ene.
101 Naming organic compounds straight chain alkanes alkenes and alkynesUnderstandingsSaturated compounds contain single bonds only and unsaturated compo. Alkenes olefins are hydrocarbons that contain a carbon-carbon double bond and are said to be unsaturated. It explains the topics using words and diagrams.
Apr 05 2014 Alkanes CnH2n2 Alkenes CnH2n Alkynes CnH2n-2 3. For example alkynes undergo many of the typical addition reactions of alkenes. This is a Power Point on naming alkenes drawing alkenes naming alkynes drawing alkynes naming cyclic compounds drawing cyclic compounds unsaturated hydrocarbons determining the parent chain of alkenes and determining the parent chain of alkynes.
Alkenes and Alkynes - PowerPoint PPT Presentation. Carbon in the chain. Alkynes are similar to alkenes in both physical and chemical properties.
Relate that to fuel and hydrocarbon so students realize the importance of hydrocarbon in the daily lives. Brainstorm as a class on some of the important things in life. Naming Hydrocarbons Alkanes Alkenes Alkynes Hook.
1 2 3 4 CH2CHCH2CH3 1-butene. PHYSICAL PROPERTIES OF ALKANES. Molecular formula C nH 2n 51.
Alkanes and Alkenes - Alkanes and Alkenes Topic 102 and 103 Alkanes have low reactivity bond enthalpies are relatively strong 348 kJ mol-1 to break a C-C bond 412 kJ mol-1 to break a C-H. Alkanes Alkenes and Polymers - Ethene can be used to make poly ethene or polythene. Choose the longest continuous chain that contains both atoms of the triple bond and number the chain to give the triple bond the lower number.
Nomenclature Organic compounds can be named either using common names or IUPAC International Union of Pure and Applied Chemistry names. If there is more than one double bond use the prefixes di- tri- tetra-. Name this Alkane 22-Dimethypropane 21.
B Sc I General Chemistry U Iii A Alkane Alkene And Alkynes

Organic Chemistry 1 Alkanes Alkenes And Alkynes Ppt Download

B Sc I General Chemistry U Iii A Alkane Alkene And Alkynes
Chapter 6 Alkenes And Alkynes Ppt Video Online Download

Chapter Thirteen Alkenes Alkynes And Aromatic Compounds Fundamentals Of General Organic And Biological Chemistry 5th Edition James E Mayhugh Oklahoma Ppt Download
Komentar
Posting Komentar